Embark on a captivating journey into the realm of unit chemical reactions, where we unravel the art of writing formula equations. Unit chemical reactions writing formula equations ws 1 opens the door to a world of chemical transformations, revealing the intricate dance of atoms and molecules.
Delve into the fundamental concepts of chemical reactions, exploring their types and the mechanisms that drive them. Discover the systematic approach to writing formula equations, ensuring accuracy and balance in representing these chemical transformations.
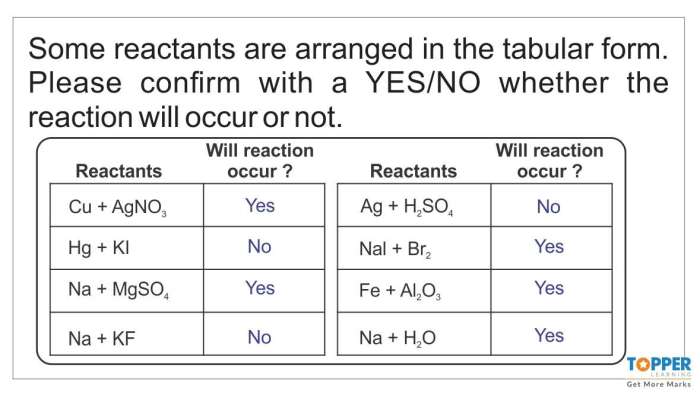
Chemical Reactions
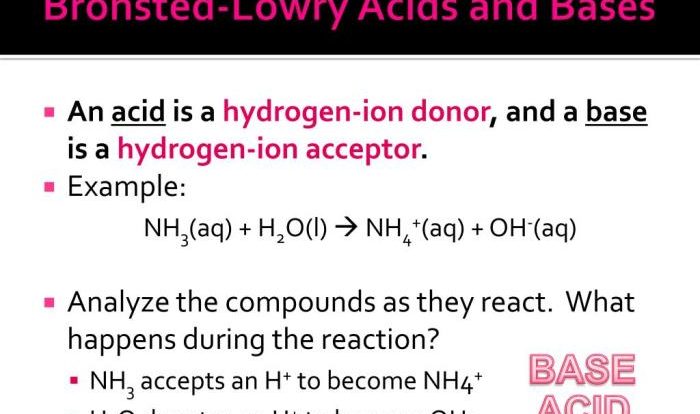
Chemical reactions are processes that involve the rearrangement of atoms and molecules. They occur when atoms or molecules interact and form new substances with different properties. Chemical reactions are essential for life and occur in all living organisms.
There are many different types of chemical reactions, including:
- Combination reactions: Two or more substances combine to form a single product.
- Decomposition reactions: A single substance breaks down into two or more products.
- Single-replacement reactions: One element replaces another element in a compound.
- Double-replacement reactions: Two compounds exchange ions to form two new compounds.
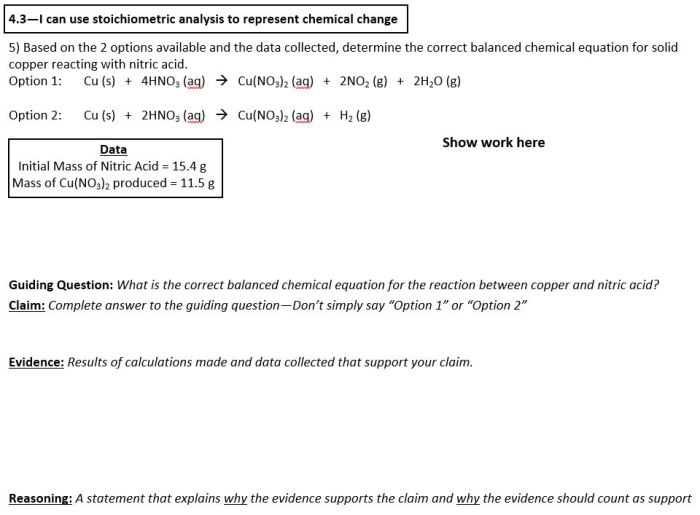
Writing Formula Equations
Formula equations are used to represent chemical reactions in a concise and symbolic way. They show the reactants and products of a reaction, as well as the stoichiometry of the reaction.
To write a formula equation, follow these steps:
- Write the chemical formulas of the reactants and products.
- Balance the equation so that the number of atoms of each element is the same on both sides.
- Add coefficients to the reactants and products to balance the equation.
For example, the following equation represents the combustion of methane:
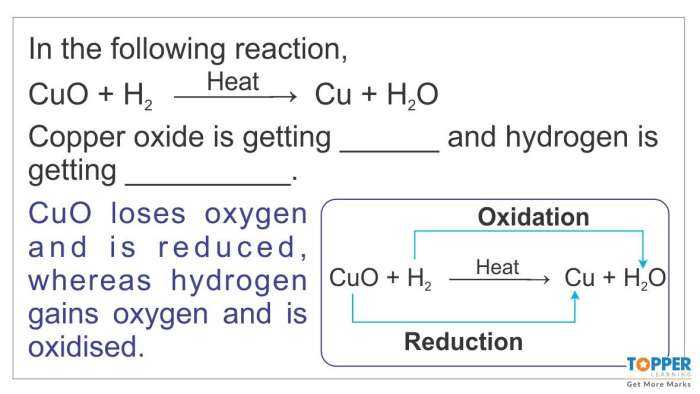
CH4+ 2O 2→ CO 2+ 2H 2O
Importance of Balancing Formula Equations
Balancing formula equations is important because it ensures that the law of conservation of mass is obeyed. This law states that mass cannot be created or destroyed in a chemical reaction. By balancing the equation, we can ensure that the total mass of the reactants is equal to the total mass of the products.
Worksheet 1, Unit chemical reactions writing formula equations ws 1
Worksheet 1 contains a number of problems that require you to write formula equations for chemical reactions. These problems will help you to practice the steps involved in writing formula equations and to understand the importance of balancing chemical equations.
To solve the problems in Worksheet 1, follow these steps:
- Read the problem carefully and identify the reactants and products.
- Write the chemical formulas of the reactants and products.
- Balance the equation so that the number of atoms of each element is the same on both sides.
- Add coefficients to the reactants and products to balance the equation.
Once you have solved the problems, check your answers against the answer key. If you have any questions, please ask your teacher or a tutor for help.
The key concepts covered in Worksheet 1 include:
- The law of conservation of mass
- The steps involved in writing formula equations
- The importance of balancing chemical equations
Key Questions Answered: Unit Chemical Reactions Writing Formula Equations Ws 1
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element is conserved on both sides of the equation, reflecting the law of conservation of mass.
How do I determine the coefficients in a balanced chemical equation?
Coefficients are adjusted until the number of atoms of each element is identical on both sides of the equation. This can be achieved through trial and error or by using algebraic methods.