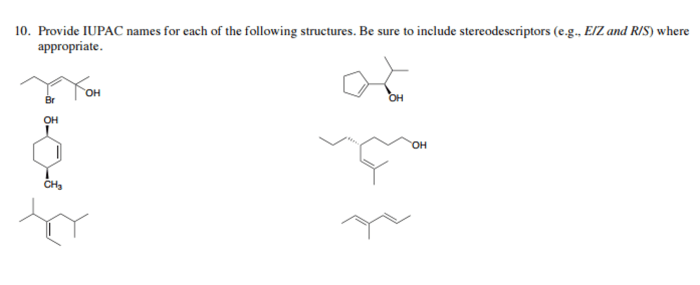
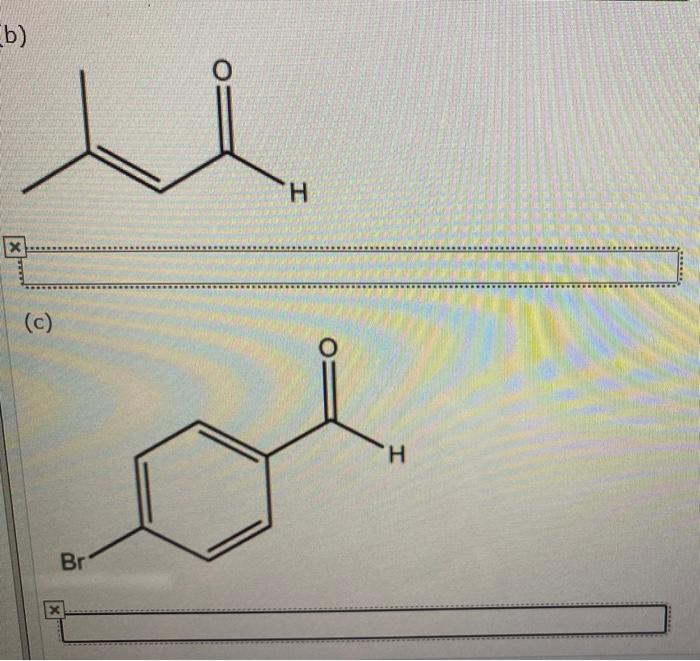
Give the IUPAC name for each of the following: This guide delves into the systematic nomenclature of organic compounds, providing a comprehensive understanding of the International Union of Pure and Applied Chemistry (IUPAC) guidelines. By exploring the principles of IUPAC nomenclature, we gain the ability to decipher and construct precise chemical names, ensuring clear communication and accurate identification of organic compounds.
The subsequent sections will illuminate the intricacies of IUPAC nomenclature, unraveling the significance of prefixes, suffixes, and locants. We will delve into the diverse realm of functional groups, examining their profound influence on the IUPAC names of compounds. Furthermore, we will encounter the concept of structural isomers, highlighting their distinct IUPAC nomenclature and emphasizing the importance of their differentiation.
IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) has established guidelines for naming organic compounds. These guidelines ensure consistency and clarity in the naming of organic compounds, regardless of their complexity.
IUPAC nomenclature uses a combination of prefixes, suffixes, and locants to identify the structure of a compound. Prefixes indicate the number of carbon atoms in the parent chain, while suffixes indicate the type of functional group present. Locants specify the position of substituents or functional groups on the parent chain.
Alkanes
Alkanes are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. The IUPAC name for an alkane is derived from the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix “-ane”. For example, the IUPAC name for the alkane with three carbon atoms is propane.
Alkenes
Alkenes are unsaturated hydrocarbons, meaning they contain at least one double bond between carbon atoms. The IUPAC name for an alkene is derived from the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix “-ene”.
The locant of the double bond is also included in the name. For example, the IUPAC name for the alkene with three carbon atoms and a double bond between the first and second carbon atoms is propene.
Alkynes, Give the iupac name for each of the following
Alkynes are unsaturated hydrocarbons, meaning they contain at least one triple bond between carbon atoms. The IUPAC name for an alkyne is derived from the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix “-yne”.
The locant of the triple bond is also included in the name. For example, the IUPAC name for the alkyne with three carbon atoms and a triple bond between the first and second carbon atoms is propyne.
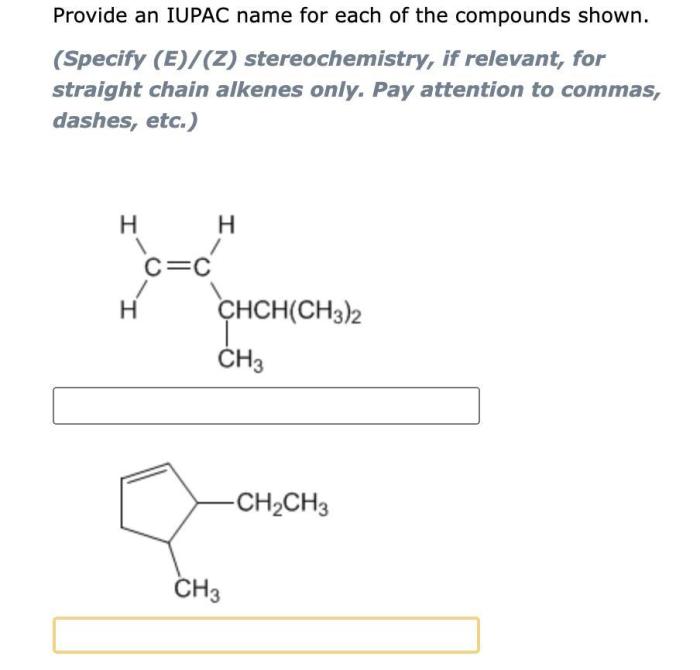
Functional Groups: Give The Iupac Name For Each Of The Following
Functional groups are specific arrangements of atoms that give organic compounds their characteristic chemical properties. Common functional groups include alcohols, aldehydes, ketones, carboxylic acids, and amines.
The presence of a functional group in a compound affects its IUPAC name. The suffix of the IUPAC name is modified to reflect the type of functional group present. For example, the IUPAC name for an alcohol is derived from the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix “-ol”.
The IUPAC name for an aldehyde is derived from the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix “-al”.
Examples
- Methanol: The IUPAC name for the alcohol with one carbon atom.
- Ethanal: The IUPAC name for the aldehyde with two carbon atoms.
- Propanone: The IUPAC name for the ketone with three carbon atoms.
- Butanoic acid: The IUPAC name for the carboxylic acid with four carbon atoms.
- Pentylamine: The IUPAC name for the amine with five carbon atoms.
Structural Isomers
Structural isomers are compounds that have the same molecular formula but different structural formulas. This means that the atoms in structural isomers are connected in different ways.
Structural isomers have different IUPAC names because the IUPAC name of a compound is based on its structure. For example, the IUPAC name for the structural isomer of butane with a branched chain is 2-methylpropane.
Importance
It is important to be able to distinguish between structural isomers because they can have different physical and chemical properties. For example, 2-methylpropane has a lower boiling point than butane.
Naming Complex Compounds
The IUPAC nomenclature rules for naming complex organic compounds are more complex than the rules for naming simple compounds. However, the same basic principles apply.
The IUPAC name for a complex organic compound is derived from the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix indicating the type of functional group present. The locants of the substituents and functional groups are also included in the name.
Challenges
The challenge in naming complex organic compounds lies in identifying the parent chain and the functional groups present. Once the parent chain and functional groups have been identified, the IUPAC name can be derived using the appropriate prefixes, suffixes, and locants.
Strategies
There are a number of strategies that can be used to name complex organic compounds. One strategy is to start with the most complex part of the molecule and work your way backwards. Another strategy is to use a table to keep track of the prefixes, suffixes, and locants that have been used.
FAQ Resource
What is the purpose of IUPAC nomenclature?
IUPAC nomenclature provides a systematic and standardized method for naming organic compounds, ensuring clarity and consistency in chemical communication.
How does the presence of functional groups affect IUPAC names?
Functional groups are specific atomic arrangements that impart characteristic properties to organic compounds. Their presence influences the IUPAC name, with prefixes and suffixes used to denote the type and location of the functional group.
What is the significance of structural isomers in IUPAC nomenclature?
Structural isomers are compounds with the same molecular formula but different structural arrangements. IUPAC nomenclature allows for the differentiation of structural isomers by assigning distinct names that reflect their unique structures.